There are many applications of systems in equilibrium in reality, and one of these system include the modern day refrigerator uses refrigeration systems to cool its contents; keeping foods cold to kill bacteria and retain freshness.

The basics of this equilibrium follows Le Chatelier’s principle and thermal equilibrium, as any refrigerant; such as ammonia, freon, and isobutene, is initially in liquid form when entering the interior of the fridge. When in the interior, the refrigerant begins to absorb the heat from the contents and change state from liquid to gas. This gas is then routed to the exterior, where the heat from the refrigerant gas is expelled to the outside surroundings. The following is a chemical equation representation of this system in equilibrium, where R is the type of refrigerant used in a refrigeration system:
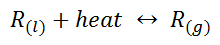 To understand this equilibrium, we must understand Le Chatelier’s principle in accordance with heat; which describes that heat is treated as a concentration and the rate of forward and reverse reactions are equal. Thus, when heat is added to the refrigerant liquid in turns into a gas and the opposite; when heat is removed the gas turns to liquid. As well as, the rate of heat withdrawn from the interior contents is equal to the rate of heat lost to the outside surroundings, which keeps the refrigerant circulating . This demonstrates a closed chemical system, where energy can pass through the system into the surroundings and vice versa, but matter cannot.
To understand this equilibrium, we must understand Le Chatelier’s principle in accordance with heat; which describes that heat is treated as a concentration and the rate of forward and reverse reactions are equal. Thus, when heat is added to the refrigerant liquid in turns into a gas and the opposite; when heat is removed the gas turns to liquid. As well as, the rate of heat withdrawn from the interior contents is equal to the rate of heat lost to the outside surroundings, which keeps the refrigerant circulating . This demonstrates a closed chemical system, where energy can pass through the system into the surroundings and vice versa, but matter cannot.

As far as society is concerned, certain refrigerants can be poisonous and damaging to health and the environment. Long ago, ammonia was initially used as a refrigerant, but due to its lethal effects on humans, was substituted for freon. However, freon has negative impacts on the Earth’s ozone layer, so isobutene is now used in current refrigerators. Furthermore, to prevent the possible leakage of refrigerant gas, the technology used (compressors, expanding valves, closed systems, etc.) is secure, and eliminates the possibility of the refrigerant coming into contact with foods and humans. Also, this technology eliminates the use of applying a refrigerant directly onto the contents. Thus, this system in equilibrium and technology of refrigeration is safe and effective in society.
Animation: http://www.pbs.org/wgbh/nova/tech/anatomy-of-a-refrigerator.html
Resources/References:
http://www.youtube.com/watch?feature=player_detailpage&v=qrcEWhurQl4
http://www.pbs.org/wgbh/nova/tech/anatomy-of-a-refrigerator.html
http://www.ior.org.uk/ior_/fantastic_fridges_site/science/how%20your%20fridge%20works.htm

my questions is about about ISO butane and Freon as a refrigerant, does heat result in a chemical change within them causing their states to change to a gas and vise versa.
Yes, yes it does. The heat is absorbed by the refrigerant from the cabin of the refrigerator, creating an absence of heat inside. The boiling point for Freon is 23.77°C, thus at room temperature Freon becomes a gas. Similarly, Isobutene has a boiling point of -7°C, thus it is more commonly used in freezers. Therefore, when there is a presence of heat within the refrigerator, that heat is drawn out by the liquid refrigerant; and results in changing state to a gas
Now I understand how refrigerants are used to cool contents, does the same apply to a freezer?
Yes, freezers work in the same manner. The difference is, refrigerant liquid is able to draw much more heat from the freezer cabin, than from the fridge cabin. Thus, temperatures in a freezer are much colder than in a refrigerator.